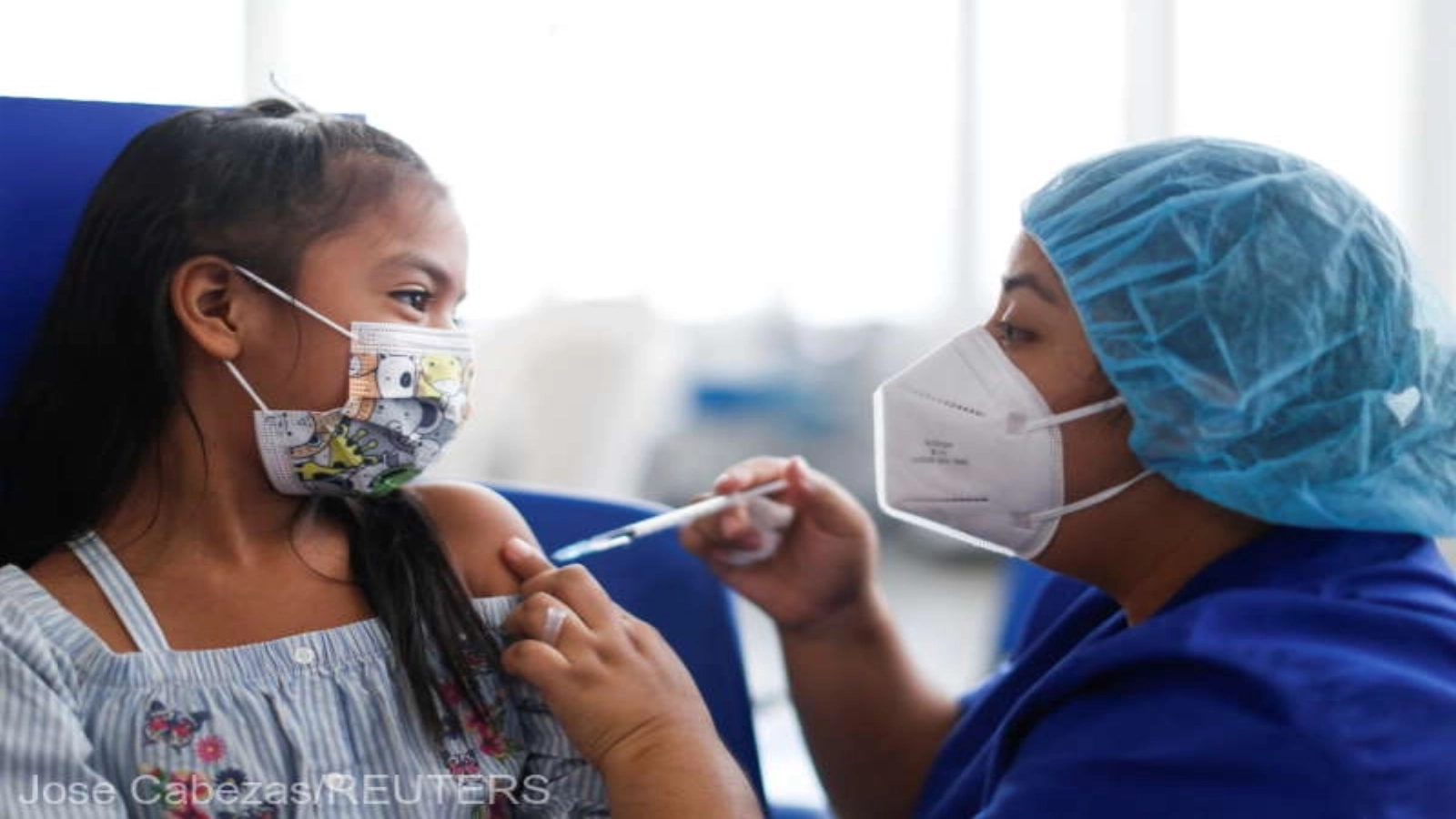
Children 5 through 11 years old can get immunised against COVID-19 in Romania from January 26, Agerpres reports.
The National COVID-19 Vaccination Coordination Committee (CNCAV) informed on Thursday how to administer the Comirnaty-Pfizer/BioNTech COVID-19 vaccine for the age group 5-11 years from January 26.
"Today, with the support of the Special Telecommunications Service, the booking platform allows the booking for vaccination of children 5 through 11 years old on January 26. The booking will be only for the vaccination centres marked as P_Ped on the platform via parent access accounts. At the moment, 219 pediatric vaccination offices are available on the booking platform nationwide. Vaccination can be carried out without prior appointment, by in-person presentation at the offices of vaccination centres designed for the vaccination of children or the offices of family physicians who have requested pediatric doses, inside the opening hours. The National Electronic Register of Vaccinations has been upgraded to allow the processing of data for this category eligible for vaccination," CNCAV said in a statement released on Thursday.
CNCAV points out that:
* the informed consent of the parents or the legal guardian of the minor is mandatory as well as undergoing medical triage by filling in the triage questionnaire designed for children; blank forms are available on the national vaccination platform;
* the Comirnaty immunisation schedule for children is 10 micrograms/dose 21 days in between; each dose is administered by the intramuscular route, similar to other eligible age groups;
* if vaccination is initiated in children turning 12 in between the two doses, the vaccination schedule will continue with the pediatric dose of 10 micrograms.
CNCAV added that the Comirnaty vaccine produced by the pharmaceutical company Pfizer/BioNTech is already approved for administration to the population over 12 years of age.
"Following the completion of the evaluation of the scientific data, the benefit-risk balance is positive, especially in children who have comorbidities and who are at increased risk of developing severe forms of the SARS-CoV-2 infection. These regulations are based on studies and recommendations that show that the immune response remains maximal for the beneficiaries of Comirnaty vaccine. A study in children 5 through 11 years old has showed that the immune response to Comirnaty administered at a lower dose (10 micrograms) is comparable to that observed at the highest dose (30 micrograms) given to those aged 16 years and over (as indicated by the anti-SARS-CoV-2 antibody levels)," CNCAV shows.
COVID-19 vaccination of children aged 5 to 11 to start in Romania on January 26
Explorează subiectul
Articole Similare

29
U.S. Senator Shaheen: House's Judiciary Committee Report on Romanian elections - erroneous
29

11
Senate amends legislation on combating harassment and violence at work
11

11
Energy Committee chairman: Distribution networks lagging behind economic growth over past three decades
11

17
Romanian Senate ratifies Romania-Ukraine agreement on emergency mutual aid
17

13
Romania's Ambassador to Bulgaria: David Popovici is a fantastic athlete, a role model for all generations
13

9
BVB stocks close Monday's trading session higher
9

12
Gambling industry's profits cannot be built on the vulnerability of children and young people (PNL's Turcan)
12

8
EnergMin Ivan: We've finished legislation to ensure gas prices will not increase after March 31
8

9
AUR Senator Peiu: Romania's current economic difficulties are unrelated to potential European recession
9

12
Ivan: Interventions at Vidraru Dam were carried out in compliance with all legal provisions
12

16
FRH President: Romania to host three major international handball tournaments in 2026
16

13
FinMin Nazare takes part in Economic and Financial Affairs Council (ECOFIN) meeting, in Brussels
13

12
USR Senator Palarie: Romania's participation in Washington Peace Council is a good thing
12
















Comentează