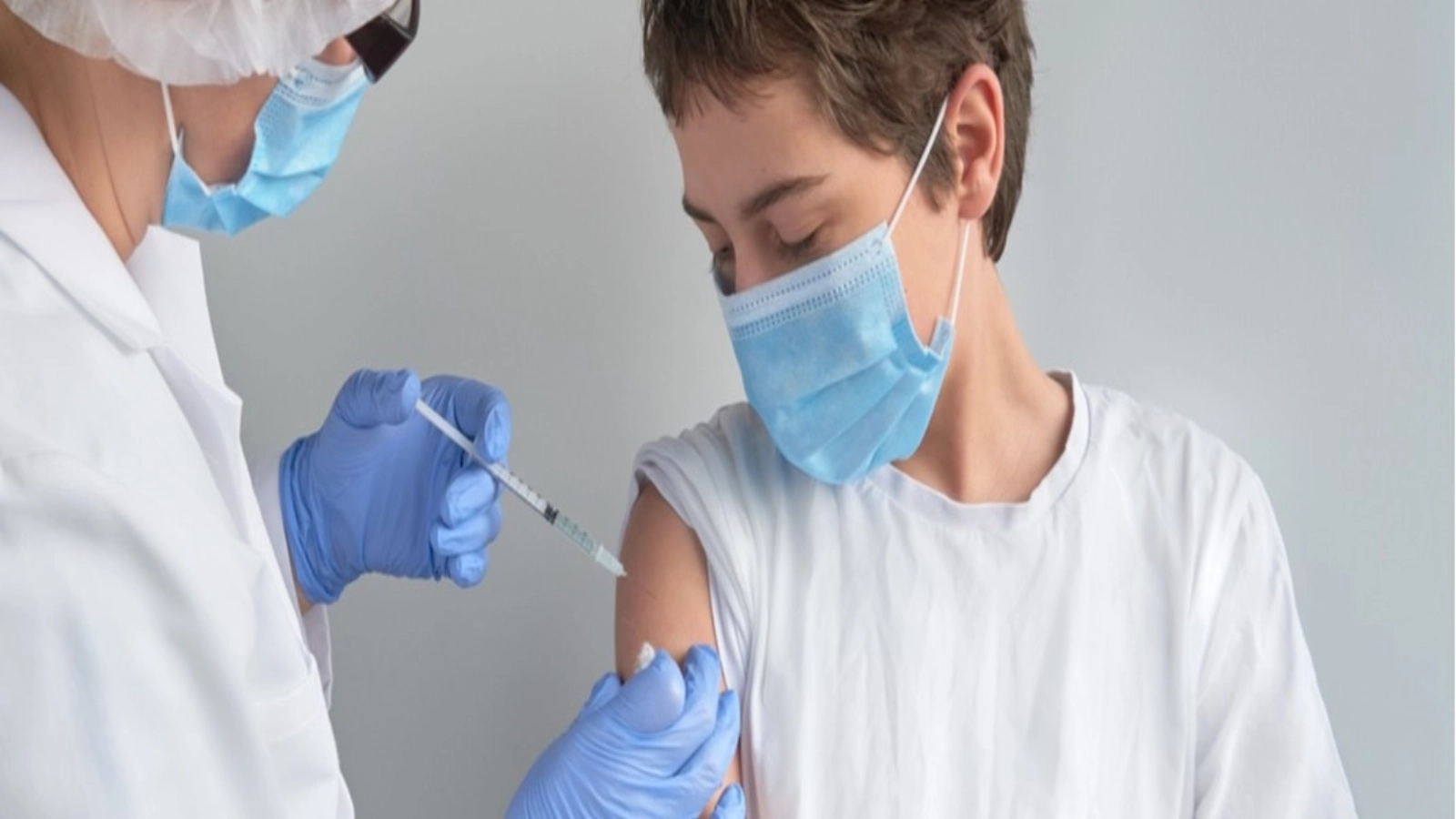
Vaccination of children in the 5-11-year age group will be "strongly recommended" for those who are registered with chronic diseases, head of Romania's National COVID-19 Vaccination Coordination Committee (CNCAV) Valeriu Gheorghita said on Tuesday.
He said that in mid-December the European Medicines Agency would make the recommendation for the vaccination of the 5-11 age group with the.
"The US Food and Drug Administration (FDA) has approved the Pfizer/BioNTech vaccine for use in children between the ages of 5 and 11. Full vaccination consists of two doses given 21 days in between, but the dosage is different as it is virtually a third of the dose currently used for those in the age group over 12 years and in adults, and from that point of view the safety and efficacy data are excellent, in that full vaccination in the 5-11 age group has been associated with an efficacy of over 91% in the prevention of COVID-19 cases. Pfizer/BioNTech has submitted clinical trial data to the European Medicines Agency, and we expect that by mid-December the European Medicines Agency to make the recommendation for those in the age group 5-11 to get the Pfizer/BioNTech vaccine," Gheorghita told a news conference on Tuesday at the Government House.
He explained that for that age group the vaccine vials are different, adding that there will be dedicated immunisation offices at the vaccination centers.
Gheorghita underscored that in particular the vaccination of children in the 5-11 age group "will be strongly recommended for those who are registered with chronic diseases., Agerpres informs.




























Comentează